Annatto: A Scarf and a Study
By Karen and Rufus Day
Karen's silk scarf
A box of annatto (Bixa orellana) arrives from Guam. The dried, fleshy seeds are the size of peppercorns and very orange. I quickly pull Dominique Cardon's Natural Dyes: Sources, Tradition, Technology and Science1 from my shelf. I know I want to dye 20/2 silk threads, but how to do it?
Cardon gives good information and references. Referencing two 1700's dyers, Macquer2 and Fernandez3, she describes making a paste with the seeds and using ash to modify the colors. My husband goes to the computer and types in the references, and to our surprise, these articles are on the web! Rufe translates the articles as I skein the silk for dyeing.

First the skeins, about 12 ounces for one scarf, are scoured in batches with 1 teaspoon of 20 Mule-team® Borax in a basin of water which is heated to simmer, kept at this temperature for 15 minutes and then cooled in the same solution and rinsed. Because I'm working with silk, I immerse the skeins in soy milk until saturated and then dried. The soy milk bath is made by soaking dried soy beans overnight, blending them, and filtering the liquid4. Now my silk is ready for color.
The annatto seeds are soaked overnight and in the morning are heated in water. To make a paste, I try to force the cooled seeds through my conical sieve with a wooden pestle, but am unable to break up the seeds enough to get a paste. I resort to using a blender, which is successful. (In the 1700s Macquer and Fernandez did not have this option.) The paste and some water are added to a basin where the skeins of silk are immersed and heated to simmer for 30 minutes. The orange skeins are hung on the tree to dry. In this way I dye all of the skeins with the annatto. However, I don't want my scarf to be a solid orange, but prefer a depth of shades.

To obtain various shades of the orange, the color must be modified in a variety of solutions. The 1700s fellows came into play again. They used ash to brighten color. I had 60 grams of Camilla wood ash to which I added 1.2 liters of boiling water, let it sit for 2 days and then decanted the liquid. I heated some of the skeins in the liquid and let it cool.

This produced a bright Halloween orange! I immersed another annatto-dyed skein in a vinegar solution which yielded a golden orange. Finally, I modified another skein of the 20/2 silk in a ferrous sulfate solution (1½ teaspoons per 2 liters water) which gave me a golden tan. The warp for my scarf was ready to weave. I prepared a weft of nubby silk, then dyed it and modified it with the iron solution. To weave the scarf I choose an eight-shaft advancing twill with a network draft (Weaver's Issue 43, Spring 1999). In the meantime, Rufus, a retired scientist, was intrigued with the effect of the ash modifier, so he began a study of the annatto on broadcloth silk.
Rufe's Study
Why does the ash produce such a bright orange? Would other modifiers affect the outcome? Does the ash contain some "secret ingredient"? Chemists know that when plant material is burnt to ash, it releases all of its carbon as carbon dioxide, and of course all of its water. The ash that remains contains mostly oxides of calcium and potassium. When these are dissolved in water they become calcium hydroxide and potassium hydroxide, both strong bases, which make solutions having high pH.
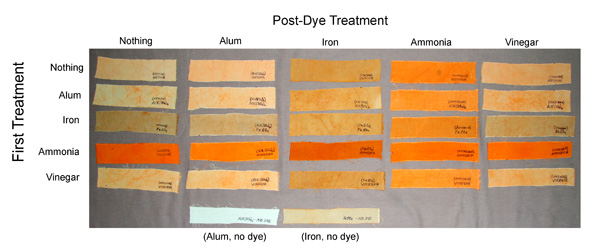
So I planned an experiment. I would test the effects of high pH (with ammonia because our wood ash was in short supply), as well as low pH (vinegar), and out of interest some modifiers, including alum (potassium aluminum sulfate) and iron (ferrous sulfate), on the color obtained with Karen's annatto preparation. First I washed 5 - 8" x10" pieces of silk (Spun Broadcloth Silk, Item 19C-000 from Exotic Silks) with soap and dried them. I soaked 2 of the pieces in 200 milliliter solutions containing either 1 gram alum (AlK(SO4)2<12H2O) or 1 gram iron (Ferrous Sulfate, FeSO4<7H2O).
After drying these, I dyed all five pieces, each in a separate bowl containing 100 milliliters of Karen's annatto preparation. The iron- and alum-treated pieces of silk were dyed this way, and the three pieces that had not been treated with iron or alum were dyed separately after adding either (i) nothing to the dye, or (ii) 50 ml 5% clear household ammonia (iii) 50 ml white vinegar to the dye. Each of the five pieces was dyed at between 140 and 195 degrees Fahrenheit for 10 minutes.
After drying, I cut each piece of the dyed silk into five strips. One of the five strips from each piece was given a post-dye treatment with nothing, one with alum, one with iron, one with ammonia, and one with vinegar. All post-dye treatments were done at the same concentrations as for dyeing, but without dye and at near boiling temperature for three to five minutes.
The results are shown in Figure 2. It is clear that dyeing with annatto in a solution with added ammonia (either with the dye or as a post-dye treatment) gives the most intense orange color, thus the "oranging" effect of the annatto that Karen observed in the silk used for her scarf is most likely due to high pH. Without post-dye treatment neither the vinegar treatment, nor the treatments with iron or alum gave any deeper orange color than did dyeing with annatto alone. But either mordanting or a post-dye treatment with iron produced a tanning effect in the annatto-dyed silk. Dyeing in the presence of ammonia followed by the iron post-dye treatment produced a deep rusty orange color. (As seen in the last row of the figure, an iron pretreatment without dye produced a light grey-tan color whereas alum alone produced very little color.)
A subsequent experiment using 20 Mule Team® Borax to produce a high pH annatto solution also produced a deep orange in the silk, supporting the idea that high pH annatto solutions give a deeper orange than neutral pH solutions.
For those interested in using it for dyeing, annatto is for sale in the Mexican section of local grocery stores under the trade name of "El Guapo." It can also be found at Amazon.com—look for "ground annatto" under "Grocery & Gourmet Food."
1 Cardon, Dominique, Natural Dyes: Sources, Tradition, Technology and Science. Archetype Publications, London, 2007. pp. 312-317.
2 Macquer, Pierre-Josef. Art de la Teinture en Soie, Paris, Desaint, 1763. (Google Books has the 1808 edition: http://books.google.com/books?id=9cIPAAAAQAAJ&dq= editions:9cIPAAAAQAAJ, pp 66-76. Annatto in French is rocou.)
3 Fernandez, L. Tratado Instructivo, y Practico Sobre el Arte de la Tintura, Blas Rombßn, Madrid, 1778. (Google Books: http://books.google.com/books?id=QUPQ50TRhLAC& printsec=frontcover&dq=Tratado+Instructivo,+y+Practico+Sobre+el+Arte+de+la+ Tintura&hl=en&ei=OcfmTNm4GY70tgPHsoWxCw&sa=X&oi=book_result&ct=result& resnum=1&ved=0CCkQ6AEwAA#v=onepage&q&f=false. Chapter 17, pp. 73-81. Annatto in Spanish is achiote.)
4 Marshall, J. at http://www.johnmarshall.to/5-EDx-SoyMilk.html
 Turkey Red Journal
Turkey Red Journal
